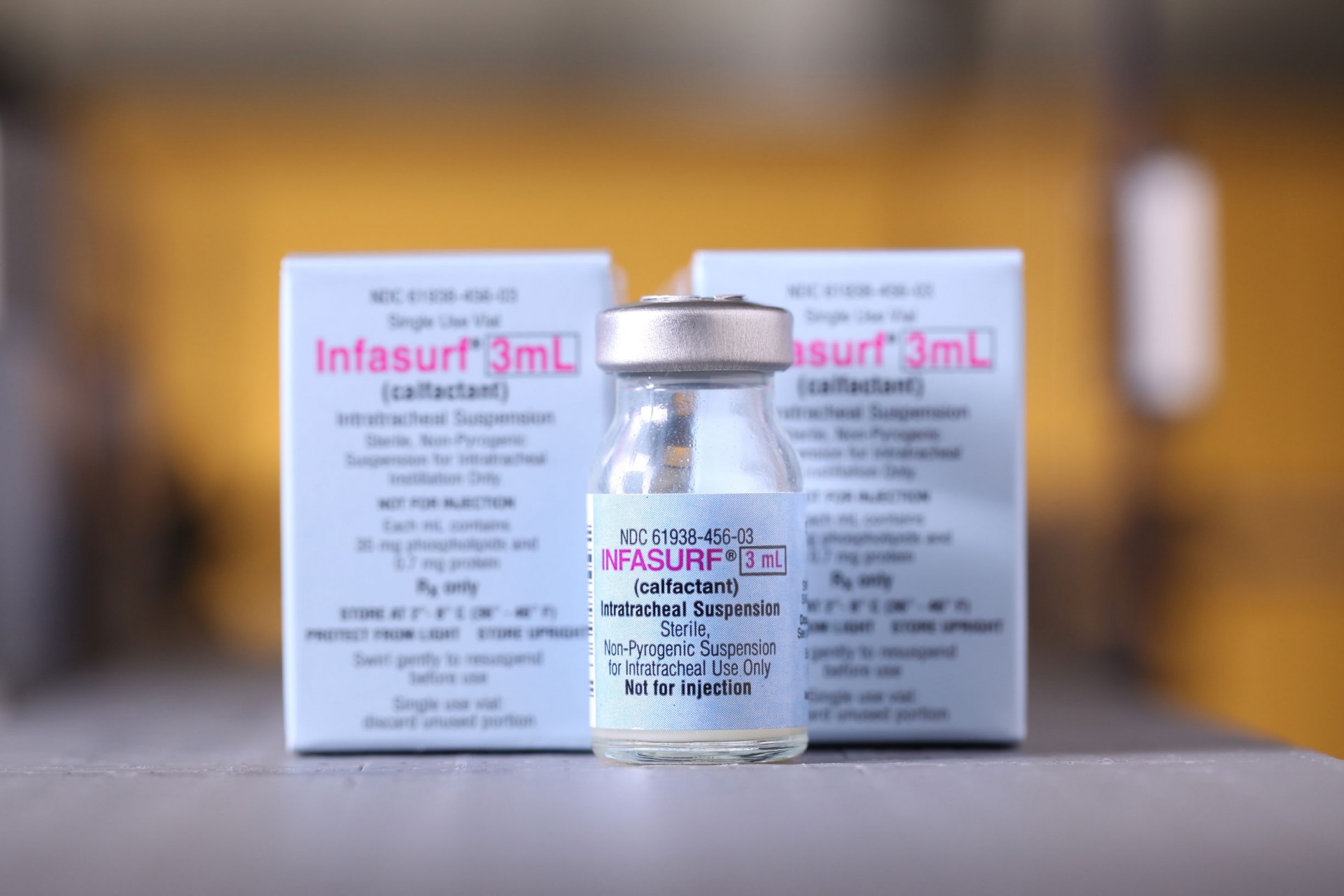
City Overseas Ltd. is proud to introduce Infasurf® (Calfactant) in Bangladesh, manufactured in the US by ONY Biotech and approved by FDA for prevention and treatment of Respiratory Distress Sydnrome in premature infants.
Infasurf® (calfactant) Intratracheal Suspension is a sterile, non-pyrogenic lung surfactant intended for intratracheal instillation only. It is an extract of natural surfactant from calf lungs which includes phospholipids, neutral lipids, and hydrophobic surfactant-associated proteins B and C (SP-B and SP-C). It contains no preservatives. Infasurf is an off-white suspension of calfactant in 0.9% aqueous sodium chloride solution. It has a pH of 5.0 – 6.2 (target pH 5.7). Each milliliter of Infasurf contains 35 mg total phospholipids (including 26 mg phosphatidylcholine of which 16 mg is disaturated phosphatidylcholine) and 0.7 mg proteins including 0.26 mg of SP-B.
The product is available in 3ml and 6ml pack sizes.
For complete details, please visit: https://infasurf.com/
Indicated for treatment of serious infections due to selected aerobic Gram-negative pathogens in patients with limited treatment options.
Read moreManufactured in the US by ONY Biotech, Infasurf® is a product approved by the US FDA for prevention and treatment of RDS in premature infants.
Read more